Neurobiology & Aging
In 2022 the department Neurobiology & Aging was joined by two technicians and a postdoc, whereas to former members left the BPRC for jobs elsewhere. The department further developed it’s work on aging and dementia, and further explored the effects of SARS-CoV-2 infections on the brain.
Background
Aging
As people age, they become more susceptible to various health conditions, including neurodegenerative diseases. Neurodegenerative diseases are a group of disorders that progressively affect the function and structure of the brain and nervous system, leading to cognitive decline and functional impairment. These diseases are often chronic, disabling, and incurable, and can significantly impact the quality of life of affected individuals and their families.
There has been a significant increase in the incidence of neurodegenerative diseases in recent years, particularly in developed countries. This increase can be attributed to several factors, including the aging of the population, changes in lifestyle, and improved diagnostic capabilities. As life expectancy continues to rise, the prevalence of neurodegenerative diseases is expected to increase further, putting a significant strain on healthcare systems and society as a whole.
Neuroinflammation
Neuroinflammation is a type of inflammation that occurs in the brain and spinal cord in response to injury, infection, or disease. Normally, inflammation is a necessary response of the immune system to protect the body from harmful stimuli, such as pathogens or toxins. However, when inflammation becomes chronic or excessive, it can lead to tissue damage and contribute to the development or progression of various diseases, including neurological disorders.
During neuroinflammation, immune cells in the brain, microglia and astrocytes, become activated and release pro-inflammatory molecules, such as cytokines and chemokines. These molecules trigger a cascade of events that can further recruit peripheral immune cells to the site of injury or damage. Additionally, neuroinflammation is associated with a wide range of neurological disorders, including Alzheimer's disease, Parkinson's disease, multiple sclerosis, and traumatic brain injury. In these conditions, neuroinflammation is thought to play a role in the degeneration of neurons and progression of disease. Additionally, aging, chronic stress, obesity, and other lifestyle factors have also been linked to increased neuroinflammation, which may contribute to cognitive decline and other symptoms. Inflammation in the brain can be regarded as a double-edged sword; it is necessary for repairing damaged tissue and fighting infections while on the other hand controlling inflammation is critical for a delicate balance between an appropriate immune response and excessive inflammation leading to collateral damage.
Although we know aging is a key contributor to the progression and aggravation of neurodegenerative diseases, it is challenging to determine their exact relationship. By analysing brain samples of different species and different ages, and their blood factors, including proteins and immune cells, we aim to get a deeper understanding of how aging may contribute to neurodegenerative diseases, but also neurological disorders due to viral infections, such as SARS-CoV-2.

Age-related blood factors
Aging comes with a lot of changes throughout the body, of which many leave an imprint in the blood composition. As previous research has shown that changes in the blood composition can have an effect on various organs, including the brain, we aimed to identify how the blood composition changes with age in humans.
To do so, we integrated the results of four human plasma proteomic datasets. By combining this information, we were able to compare age-associated changes of ~5000 proteins in the plasma of individuals between the age of 16 and 100. Across these datasets, we identified a set of Aging Proteins (APs) which showed similar age-associated effects and validated these effects in other independent studies and methods. APs were found to be highly associated with various age-related diseases, such as organ failure and dementia. Moreover, the expression levels of these APs in the plasma were found to be good predictors of chronological age, even across independent datasets. However, some individuals showed a discrepancy between their chronological age and estimated biological age based on their plasma proteome. To further investigate this difference we identified several candidate proteins that may contribute to the acceleration of the aging process, or may actually slow it down. These results will be published in 2023.
Currently, we are translating and validating our findings in the Rhesus Macaque (Macaca Mulatta). We performed proteomics on plasma samples from our own macaque colony spanning a broad age range, and identified similarities in several human APs and the aging Macaque plasma proteome. To gain a better understanding of brain aging, we selected APs most likely reflective of this process as follow-up candidates to compare transcriptomic and proteomic changes in brain tissue. Using this approach we aim to gain a better mechanistic understanding of brain aging, which may ultimately lead to novel therapies to remain healthy and cognitively fit for as long as possible.
TO TOP ^ < BACK << HOME
Aging brain collection
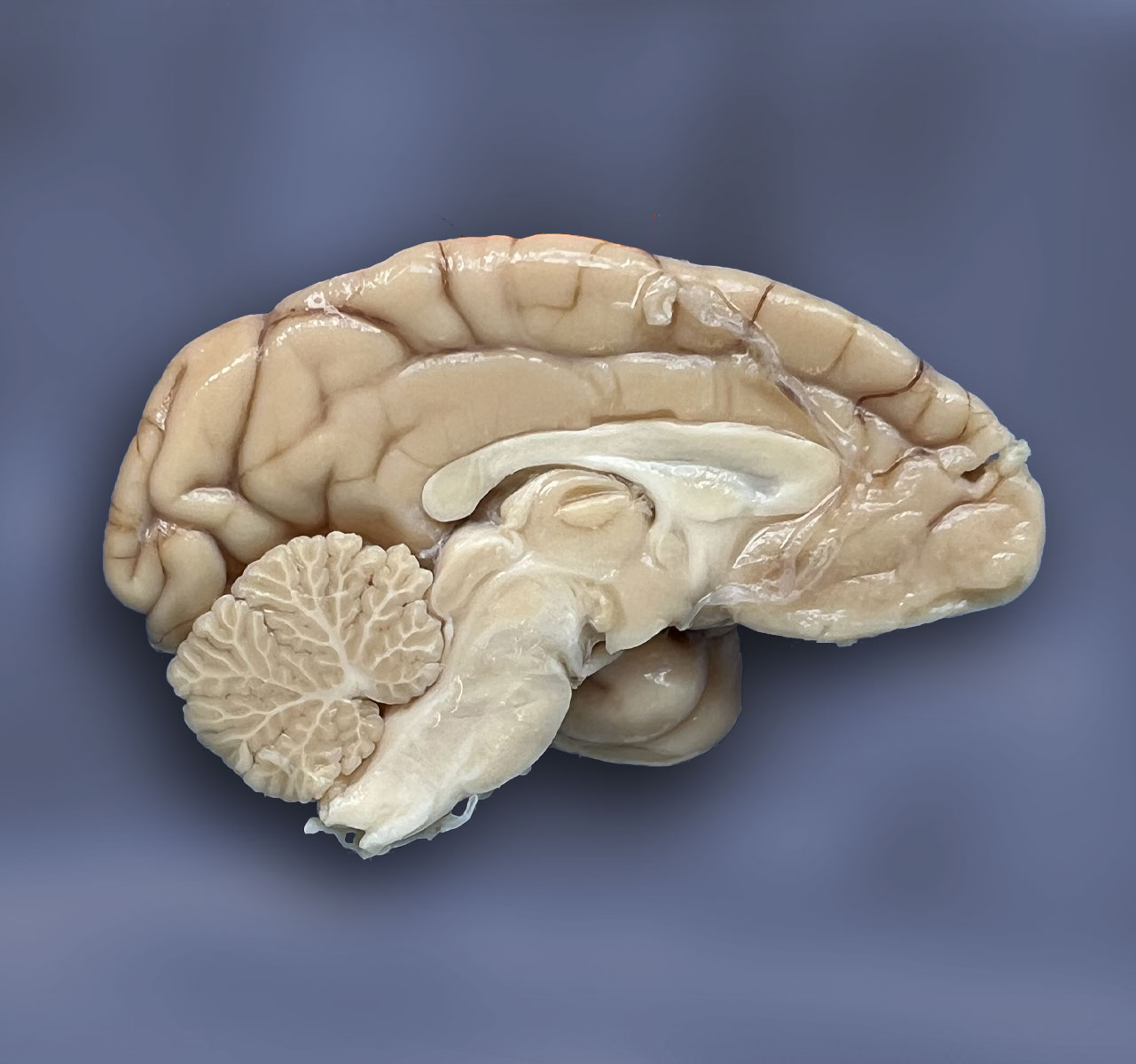
To study various aspects of brain aging, we have started to collect, dissect, and store the brains of non-human primates in a structural manner. With this method we are currently setting up a biobank of invaluable brain material for research on the aging brain and neurodegenerative disease. This material can be used for research interests of the BPRC but can also be utilised by external parties for research collaborations.
Over the last year we have dissected 75 brains and stored over 2700 different blocks, both snap frozen as well as formalin fixed paraffin embedded (FFPE). The brain biobank is realised from our current efforts to collect as many brains as possible from the NHPs in the BPRC. In this way we are generating a collection that contains the brains of normal aging NHPs but also brains of NHPs during disease.
Research topics using the aging brain collection:
Neuroinflammation
One of the research areas that is currently being focused on is the heterogeneity of microglia during aging. Microglia are the resident immune cells of the central nervous system and play a critical role in the maintenance of brain homeostasis and are the main driving force of inflammatory processes. Over the years, research has revealed that microglia are not a uniform population of cells, but rather a heterogeneous group with diverse functions and properties. Microglia can vary in morphology, gene expression, and response to stimuli depending on their location within the brain, developmental stage, and disease state. Understanding the heterogeneity of microglia is important for developing targeted therapies for neurological disorders and improving our understanding of the complex interactions between microglia and other cells in the brain. For this we are analysing the morphology and states of microglia throughout the lifespan in NHPs by utilising the blocks generated in our brain biobank. We have set up a pipeline for microglial morphology analysis to analyse many different parameters regarding the complexity and the shape of microglial cells.
Neuropathology
Due to the increase in the incidence of neurodegenerative disease we are investigating whether aging NHPs develop similar pathology in the central nervous system that is also observed in humans. Neurodegenerative diseases are characterized by pathology in the brain, for example Alzheimer’s disease is accompanied by the formation of Aβ plaques and tau tangles in the cortex. As these diseases progress, the pathology spreads throughout the central nervous system leading to neurological complications and loss of quality of life. To establish whether NHPs can be used for research on neurodegenerative diseases we have started to investigate if pathology associated with ageing and neurodegenerative diseases can be found in ageing macaques.
Overall, we aim to determine how similiar aging processes are between non-human primates and humans.
TO TOP ^ < BACK << HOME
SARS-CoV-2 induced neuroinflammation and neurological consequences

Coronavirus disease 2019 (COVID-19) patients initially develop respiratory symptoms, but they may also suffer from neurological symptoms. People with long-lasting effects after acute infections with severe respiratory syndrome coronavirus 2 (SARS-CoV-2), i.e., post-COVID syndrome or long COVID, may experience a variety of neurological manifestations. Although we do not fully understand how SARS-CoV-2 affects the brain, neuroinflammation likely plays a role.
In 2022 we published a paper on our first SARS-CoV-2 infection study in which we describe neuroinflammatory processes in the brains of four cynomolgous and four rhesus macaques infected with the Alpha variant of SARS-CoV-2.
We continued to investigate the effects of SARS-CoV-2 infection on the brain in four rhesus macaques that were infected with the Delta variant. Because neuroinflammation was apparent in the first SARS-CoV-2 study we imaged these four macaques longitudinally with a PET-CT tracer more specific to neuroinflammation, namely [18F]DPA-714, which targets the translocator protein TSPO. This study revealed an increased TSPO tracer uptake throughout the brain of all infected animals already from 2 days post infection until approximately 30 days post infection, suggesting neuroinflammation is an active and continuous process even weeks after the acute infection period. Postmortem immunohistochemical analysis showed increased TSPO expression and a clear response of the glial cells in various brain regions of SARS-CoV-2 infected animals. These results will be published in 2023. We are currently further exploring SARS-CoV-2 tropism, the mechanism behind these neuroinflammatory processes, and the neurodegenerative consequences.
TO TOP ^ < BACK << HOME


